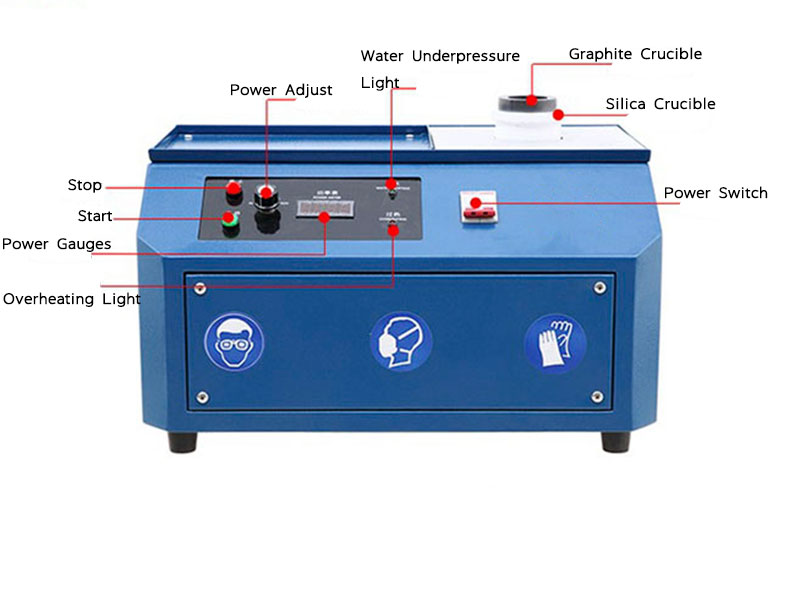
purification process of gold
Understanding the Purification Process of Gold
The purification process of gold is an essential procedure that transforms raw gold ore into a refined and pure metal. This process removes impurities, allowing the gold to reach high levels of purity, which is critical for various applications, including jewelry, electronics, and investment. The process involves several methods, each offering unique advantages based on the desired level of purity, the quantity of gold, and the nature of the impurities. This article explores the most common techniques used in gold purification, highlighting how they contribute to achieving high-quality, refined gold.
Why Gold Needs Purification
Gold, in its natural form, is often mixed with other metals such as copper, silver, or iron. These impurities lower the quality of the gold and can hinder its use in specific industries. The purification process of gold ensures that the metal reaches the highest possible purity standards, typically between 99.5% to 99.999%, depending on the method used.
For industrial purposes, such as electronics and high-precision equipment, gold must be extremely pure to ensure performance and durability. Similarly, in the jewelry industry, gold’s aesthetic value depends on its purity. Thus, the process of purification is crucial to maximize the metal’s worth and utility.
Common Methods for the Purification Process of Gold
Fire Assaying
One of the oldest methods used in the purification process of gold is fire assaying. This technique has been practiced for centuries and remains relevant due to its accuracy in determining the purity of gold.
- Process: Gold is heated to extremely high temperatures in a furnace. A flux, often made from lead, is added to bind with the impurities. The molten mixture is then poured, and the pure gold settles at the bottom while the impurities rise to the surface, forming a slag that can be removed.
- Advantages: Fire assaying is highly reliable for separating gold from other metals and provides an accurate measurement of gold content, especially in ores.
Miller Process
The Miller process is widely used in large refineries as a first step in purifying gold. It involves blowing chlorine gas through molten gold to remove impurities.
- Process: The chlorine gas reacts with other metals, such as silver and copper, forming chlorides that rise to the surface and can be skimmed off. This leaves behind gold that is 99.5% pure.
- Advantages: The Miller process is efficient and suitable for large-scale operations. However, to achieve higher purity, it is often followed by other methods, such as the Wohlwill process.
Wohlwill Process
The Wohlwill process is an electrolytic method that produces extremely pure gold, often reaching 99.999% purity. It is commonly used after the Miller process to refine gold further.
- Process: The gold is placed in an electrolyte solution of gold chloride. When an electrical current is passed through the solution, pure gold is deposited onto a cathode, while the impurities remain in the solution.
- Advantages: The Wohlwill process is highly accurate and can produce the purest gold possible. It is the preferred method for creating investment-grade gold.
Aqua Regia
Aqua regia is a chemical refining method that involves using a mixture of nitric acid and hydrochloric acid. This process is effective in dissolving gold and separating it from other metals.
- Process: Gold is dissolved in the acid mixture, and impurities either remain undissolved or are filtered out. The gold is then precipitated from the solution using a reducing agent, typically sodium metabisulfite.
- Advantages: Aqua regia is particularly useful for refining small quantities of gold. It can produce high-purity gold but is usually more labor-intensive than other methods.
Electrolytic Refining
Electrolytic refining is a modern technique widely used for the purification process of gold. It uses an electrical current to dissolve gold into a solution, where it is then deposited onto a cathode as pure gold.
- Process: A gold anode is placed in an electrolyte solution, and when an electric current is applied, the gold dissolves. Impurities either remain in the solution or form a sludge. The pure gold is collected at the cathode.
- Advantages: Electrolytic refining is highly efficient and can be used to produce gold with a purity of 99.99%. It is ideal for refining large quantities of gold and is used by major refineries worldwide.
The Role of Purity in Gold’s Value
The value of gold increases significantly with its level of purity. The purification process of gold directly affects the price that the metal can fetch in the market. For example, investment-grade gold, often used to create gold bars and coins, must be of the highest purity to maintain its value. Industrial gold, especially in electronics, also needs to be free from impurities to ensure its functionality in sensitive components.
Jewelry made from highly purified gold tends to be more valuable and is sought after for its brilliance and color. As a result, gold purification plays an essential role in determining the metal’s worth and how it can be utilized across industries.
Environmental Considerations
While the purification process of gold is vital for producing high-quality gold, it also poses environmental challenges. Some methods, such as the use of aqua regia or other chemicals, can produce hazardous waste. Proper disposal of these chemicals is necessary to prevent environmental contamination.
Additionally, gold mining and the subsequent purification processes can have a significant impact on the environment. The energy consumption involved in methods like fire assaying and electrolytic refining is considerable, and efforts to reduce this environmental footprint are ongoing.
The purification process of gold is a critical component of refining this precious metal, ensuring that it meets the highest standards of purity for various applications. From traditional methods like fire assaying to modern techniques such as electrolytic refining, each method plays a vital role in producing gold that is valuable and functional. As the demand for pure gold continues to grow, refining technologies will evolve to become more efficient, environmentally friendly, and capable of achieving even higher purity levels.